|
|
|
|
|
|
|
News & Views item - January 2011 |
![]() For the First N&V of the Millennium's Second Decade Spare a Thought for the Weak
Photon. (January 2, 2011)
For the First N&V of the Millennium's Second Decade Spare a Thought for the Weak
Photon. (January 2, 2011)
Bob Park continues his crusade against stupidity one-hundred and six years after the deduction of the photoelectric effect.
 PHOTONS:
WHAT ALBERT EINSTEIN KNEW ABOUT CELL-PHONE RADIATION.
PHOTONS:
WHAT ALBERT EINSTEIN KNEW ABOUT CELL-PHONE RADIATION.
Maybe I missed it, but I have seen nothing from major media sources refuting the
preposterous claim that radiation from cell phones and other wireless devices is
linked to human health problems. We are bathed in microwave radiation. Most of
it is as natural as sunshine, but wireless communication, including cell phone
radiation, is not. What do we know about the effect of this stuff on the human
body, and how long ago did we know it? The starting point is 1905, sometimes
called "Albert
Einstein's miracle year." One of the four "miracle" papers he
published that year dealt with the photoelectric effect. He treated the light
striking an object as particles called quanta, having energy equal to the
frequency times the Planck constant*. This predicted a photoelectron threshold at
the extreme blue end of the visible spectrum, below which there would be no
photoemission. Almost nobody believed him, including Robert Millikan, perhaps
the world's greatest experimentalist. The photoelectric effect had already been
explained with Maxwell’s wave theory, but experimental confirmation was lacking.
Einstein wasn't bothered; he had other great things to do while waiting for
confirmation. Millikan did the experiment in 1917; it agreed perfectly with
Einstein's theory. The 1921 Nobel Prize in Physics was awarded to Einstein for
his theory of the photoelectric effect. Millikan won the Prize two years
later. Their results show that microwaves are great for warming pizza and
they don't cause cancer.
________________________________________________________________________________________________
*Credit Wikipedia: The Planck constant has dimensions of energy multiplied by time, which are also the dimensions of action. In SI units, the Planck constant is expressed in joule seconds (J·s). The dimensions may also be written as momentum multiplied by distance (N·m·s), which are also the dimensions of angular momentum.
The value of the Planck constant is:[1]
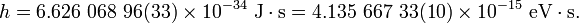
The value of the reduced Planck constant is:
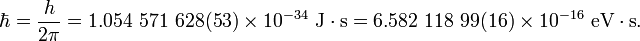
The two digits between the parentheses denote the
standard uncertainty in the last two digits of the value. The figures cited
here are the 2006
CODATA recommended values for the constants and their uncertainties. The
2006 CODATA results were made available in March 2007 and represent the
best-known, internationally-accepted values for these constants, based on all
data available as of 31 December 2006. New CODATA figures are scheduled to be
published approximately every four years.