|
|
|
|
|
|
|
News & Views item - October 2009 |
![]() 2009 Nobel Prize for Chemistry Awarded to Venkatraman Ramakrishnan, Thomas
Steitz and Ada Yonath. (October 8, 2009)
2009 Nobel Prize for Chemistry Awarded to Venkatraman Ramakrishnan, Thomas
Steitz and Ada Yonath. (October 8, 2009)
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry for 2009 jointly to Venkatraman Ramakrishnan, Thomas A. Steitz and Ada E. Yonath "for studies of the structure and function of the ribosome"
 Venkatraman
Ramakrishnan, US citizen. Born in 1952 in Chidambaram,
Tamil Nadu, India. Ph.D. in Physics in 1976 from Ohio University, USA.
Senior Scientist and Group Leader at Structural Studies Division, MRC
Laboratory of Molecular Biology, Cambridge, UK. Venkatraman
Ramakrishnan, US citizen. Born in 1952 in Chidambaram,
Tamil Nadu, India. Ph.D. in Physics in 1976 from Ohio University, USA.
Senior Scientist and Group Leader at Structural Studies Division, MRC
Laboratory of Molecular Biology, Cambridge, UK. |
 Thomas
A. Steitz, US citizen. Born in 1940 in Milwaukee, WI, USA.
Ph.D. in Molecular Biology and Biochemistry in 1966 from Harvard
University, MA, USA. Sterling Professor of Molecular Biophysics and
Biochemistry and Howard Hughes Medical Institute Investigator, both at
Yale University, CT, USA. Thomas
A. Steitz, US citizen. Born in 1940 in Milwaukee, WI, USA.
Ph.D. in Molecular Biology and Biochemistry in 1966 from Harvard
University, MA, USA. Sterling Professor of Molecular Biophysics and
Biochemistry and Howard Hughes Medical Institute Investigator, both at
Yale University, CT, USA. |
 Ada
E. Yonath, Israeli citizen. Born in 1939 in Jerusalem,
Israel. Ph.D. in X-ray Crystallography in 1968 from the Weizmann
Institute of Science, Israel. Martin S. and Helen Kimmel Professor of
Structural Biology and Director of Helen & Milton A. Kimmelman Center
for Biomolecular Structure & Assembly, both at Weizmann Institute of
Science, Rehovot, Israel. Ada
E. Yonath, Israeli citizen. Born in 1939 in Jerusalem,
Israel. Ph.D. in X-ray Crystallography in 1968 from the Weizmann
Institute of Science, Israel. Martin S. and Helen Kimmel Professor of
Structural Biology and Director of Helen & Milton A. Kimmelman Center
for Biomolecular Structure & Assembly, both at Weizmann Institute of
Science, Rehovot, Israel. |
The Royal Swedish Academy of Sciences in explaining its decision said in part:
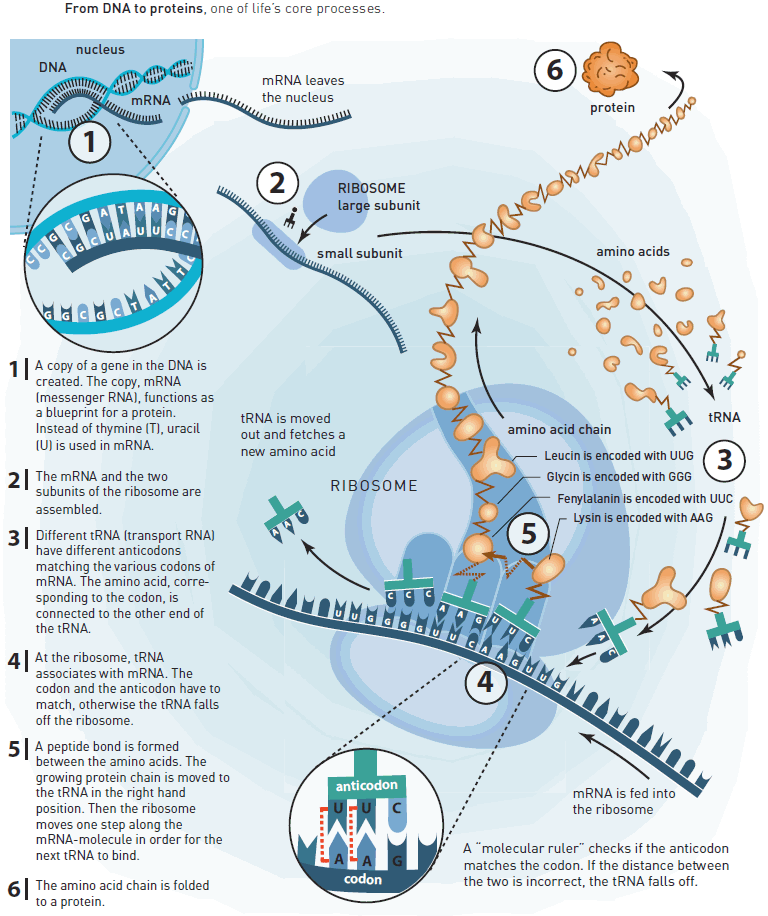
The Nobel Prize in Chemistry for 2009 awards studies of one of life's core processes: the ribosome's translation of DNA information into life. Ribosomes produce proteins, which in turn control the chemistry in all living organisms. As ribosomes are crucial to life, they are also a major target for new antibiotics.
This year's Nobel Prize in Chemistry awards Venkatraman Ramakrishnan, Thomas A. Steitz and Ada E. Yonath for having showed what the ribosome looks like and how it functions at the atomic level. All three have used a method called X-ray crystallography to map the position for each and every one of the hundreds of thousands of atoms that make up the ribosome.
An understanding of the ribosome's innermost workings is important for a scientific understanding of life. This knowledge can be put to a practical and immediate use; many of today's antibiotics cure various diseases by blocking the function of bacterial ribosomes. Without functional ribosomes, bacteria cannot survive. This is why ribosomes are such an important target for new antibiotics.
Robert Service in reporting on the award writes in ScienceNow: "The three groups have also begun to push practical applications of their work. All three, for example, have reported crystal structures that show how different antibiotics bind to the ribosome. And several companies are now using these structures in an effort to design new antibiotics against worrisome infections, such as methicillin-resistant Staphylococcus aureus and tuberculosis.
"But Steitz, for one, says he never thought initially that anything more than a fundamental insight into the molecular workings of biology would come of the work. 'It seemed a bit like trying to climb Mount Everest,' Steitz says. 'We knew it was doable. But we didn't know how to get there. When we got there in 2000, it was exhilarating. In fact, it was the most exhilarating moment I've had in science.'"
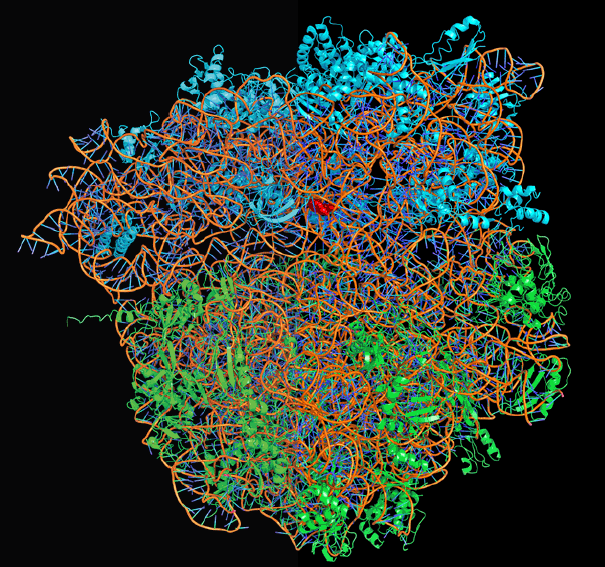
Computer generated model of the ribosome based on X-ray
crystallographic data